Transporters

Transporter Assays
Transporters are membrane-bound proteins that enable movement of substances, from nutrients to xenobiotics, in and out of cells. Drug transporter interactions are an important part of the pharmacokinetic properties of drug products. Understanding which transporters drive influx/efflux of your drug can help predict in vivo safety and performance, optimise development, and speed up regulatory approval.
Drug transporters are grouped into two classes:
ATP binding cassette (ABC)
Transporters that are relevant for intestinal absorption, urinary excretion and blood-brain barrier transfer. Examples include P-glycoprotein (P-gp) and BCRP.
Solute Carrier (SLC)
Transporters that mainly impact renal and hepatic uptake of drugs, as well as urinary excretion. Examples include OATP1B1 and OCT1
Pharmaron has extensive experience evaluating the potential interactions of compounds with drug transporter proteins. We have years of experience with in vitro transporter substrate and inhibitor potential assay development. Our expertise spans co-medications, product profiles, development plans, clearance routes, chemical structures, and physicochemical properties.
Our suite of in vitro assays enables robust and comprehensive characterization of drug interactions with both ABC and SLC transporters. The cell lines and inhibitors we use align with all FDA recommendations for these assays.
Other Express Services
Project Deliverables:
- Assay results shared in 5-10 business days
- Report shared through the secure portal
- Data archived in 21 CFR Part 11 compliant portal for posterity
- Easy check out and payment with credit card (Visa, Mastercard, AmEx) or Purchase Order
- Spend more than $25,000 within a calendar year to unlock an additional 10% discount, valid for online purchases only
Available Express Transporter Assays
Inhibition
This assay is used to screen for inhibition of P-gp by a test article, by measuring its effect on the bidirectional permeability of a P-gp substrate through Caco-2 cell monolayers.
Required from Sponsor
- Either a minimum of 100 µL of test article at 10 mM in DMSO or 2 mg of powder
- Molecular weight of the test article and its salt form
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Cell batch QC results
- The percent recovery of digoxin from the assay wells containing Caco-2 monolayers
- The apparent permeability (Papp) of digoxin in both directions, in the presence and absence of inhibitor
- The efflux ratio [(Papp B to A)/(Papp A to B)] of digoxin in the presence and absence of inhibitor
- P-gp substrate classification:
- Positive: Digoxin efflux ratio ≥ 2.0 in the absence of inhibitor and corrected efflux ratio reduced by ≥ 50% in the presence of inhibitor
- Negative: Digoxin efflux ratio ≥ 2.0 in the absence of inhibitor and corrected efflux ratio reduced by <50% in the presence of inhibitor
Substrate
- Test article and digoxin each at 10 µM in HBSSg with maximum DMSO concentration ≤ 0.8%
Assay System
- Confluent monolayers of Caco-2 (clone C2BBe1) cells, 21-28 days old
Assay Conditions
- Bidirectional permeability of Digoxin in Caco-2 cells in the presence and absence of inhibitor
- Transport buffer: HBSSg, pH 7.4 ± 0.2
- Apical and basolateral side at pH 7.4
- Dose two cell monolayers for each direction (n=2), in the presence and absence of inhibitor
- Dose apical side for (A→B) assessment
- Dose basolateral side for (B→A) assessment
- Sample from both apical and basolateral sides at 120 minutes
- Determine the concentrations of digoxin using a generic LC-MS/MS method with a minimum 6-point calibration curve
Assay QC
- The quality of the monolayer batch is verified using control compounds before the monolayers are released for use
- The quality of each monolayer used in the assay is verified by calculating the Papp for the control article, lucifer yellow, dosed post-experimentally
Notes
- The results from this assay are sent to the sponsor in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference articles.
- The solubility of the test article in Hanks’ buffer containing ≤ 0.8% DMSO must be greater than the test concentration. If the solubility of the test article is unavailable, Absorption Systems can conduct a solubility assessment at an additional charge.
- Assay conditions with the inhibitor include:
- A 30 minute pre-incubation with test article
- Assay conditions without the inhibitor includes
- A 30 minute pre-incubation with buffer
- Digoxin can be run in the presence and absence of Valspodar as an optional positive control
- Valspodar dosed at 1 µM
This MDR assay is used to screen for inhibition of P-gp by a test compound, by measuring its effect on the bidirectional permeability of a P-gp substrate through MDR1-MDCK cell monolayers.
Required from Customer
- Either a minimum of 300 µL of test compound at 10 mM in DMSO, or 5 mg of powder
- Exact molecular mass of test compound and its salt form
- Solubility and chemical stability of the test compound in Hanks’ buffer
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Apparent permeability (Papp) of digoxin in both directions, in the presence and absence of test compound
- Efflux ratio [(Papp B to A)/(Papp A to B)] of digoxin in the presence and absence of test compound
- Classification of test compound as a likely inhibitor or non-inhibitor of P-gp at the concentration tested
Substrate
- Test compound and digoxin each at 10 µM in modified Hanks’ buffer (HBSSg) with final DMSO concentration < 0.8%
Assay System
- Confluent monolayers of MDR1-MDCK cells, 7-14 days old, in Transwell® dual-chamber plates, with apical and basolateral pH 7.4
Assay Conditions
- Dose duplicate monolayers (N=2) on apical side for A to B digoxin permeability
- Dose duplicate monolayers (N=2) on basolateral side for B to A digoxin permeability
- Repeat above steps on monolayers pretreated and co-dosed with test compound on both apical and basolateral sides
- Sample both apical and basolateral sides at 120 minutes
- Determine the concentration of digoxin using a generic LC-MS/MS method with a minimum 4-point calibration curve
Assay QC
- Verify the quality of the cell monolayer batch using control compounds before the monolayers are released for use
- Verify the quality of each monolayer used in an assay by a pre-experiment TEER measurement and by calculating the Papp for the co-dosed control compound, Lucifer yellow
Notes
- The results from this assay are provided to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- The solubility of the test compound in Hanks’ buffer containing < 0.8% DMSO must be greater than the test concentration. If the solubility of the test compound is unavailable, Absorption Systems can conduct a solubility assessment at an additional charge.
Options
The customer can request that a positive control be performed in parallel (additional fees apply)
This BCRP transporter assay is used to screen for inhibition of BCRP by a test compound, by measuring its effect on the bidirectional permeability of a BCRP substrate through BCRP-MDCK cell monolayers
Required from Customer
- Either a minimum of 300 µL of test compound at 10 mM in DMSO, or 5 mg of powder
- Exact molecular mass of test compound and its salt form
- Solubility and chemical stability of the test compound in Hanks’ buffer
- MSDS or handling and storage information,
e.g., light sensitive, store at -20°C, etc.
Deliverables
- Apparent permeability (Papp) of cladribine in both directions, in the presence and absence of test compound
- Efflux ratio [(Papp B to A)/(Papp A to B)] of cladribine in the presence and absence of test compound
- Classification of test compound as a likely inhibitor or non-inhibitor of BCRP at the concentration tested
Substrate
- Test compound and cladribine each at 10 µM in modified Hanks’ buffer (HBSSg) with final DMSO concentration < 0.8%
Assay System
- Confluent monolayers of BCRP-MDCK cells, 7-14 days old, in Transwell® dual-chamber plates, with apical and basolateral pH 7.4
Assay Conditions
- Dose duplicate monolayers (N=2) on apical side for A to B cladribine permeability
- Dose duplicate monolayers (N=2) on basolateral side for B to A cladribine permeability
- Repeat above steps on monolayers pretreated and co-dosed with test compound on both apical and basolateral sides
- Sample both apical and basolateral sides at 120 minutes
- Determine the concentration of cladribine using a generic LC-MS/MS method with a minimum 4-point calibration curve
Assay QC
- Verify the quality of the cell monolayer batch using control compounds before the monolayers are released for use
- Verify the quality of each monolayer used in an assay by a pre-experiment TEER measurement and by calculating the Papp for the co-dosed control compound, Lucifer yellow
Notes
- The results from this assay are provided to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- The solubility of the test compound in Hanks’ buffer containing < 0.8% DMSO must be greater than the test concentration. If the solubility of the test compound is unavailable, Absorption Systems can conduct a solubility assessment at an additional charge.
Options
The customer can request that a positive control be performed in parallel (additional fees apply)
This OAT1 transporter assay is used to screen for inhibition of OAT1 by a test compound in OAT1-transfected HEK293 cells at a single concentration
Required from Customer
- Either a minimum of 300 µL of test compound at 10 mM in DMSO, or 5 mg of powder
- Exact molecular mass of test compound and its salt form
- Relevant solubility of the test compound
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Normalized uptake of para-aminohippurate (PAH) in the presence and absence of test compound in OAT1-transfected and vector control cells
- Percent inhibition by the test compound
- >50% inhibition indicates that the test compound is a significant inhibitor of OAT1
Substrate
- OAT1 probe substrate PAH at 10 μM
- Test compound at 10 μM in modified Hanks’ buffer with less than 0.8% DMSO
Assay System
- OAT1-transfected HEK293 cells and vector control cells cultured in 24-well plates
Assay Conditions
- Measure uptake of PAH in OAT1-transfected and vector control cells with and without test compound
- Single concentration of test compound
- Two cell lines: OAT1-transfected and vector control cell lines
- Four treatments as follows:
- OAT1-transfected cells with PAH +/-test compound
- Vector control cells with PAH +/- test compound
- Perform assay in duplicate (N=2 per treatment)
- Wells are equilibrated with blank buffer containing 1% BSA prior to initiating the assay
- Buffer is aspirated and replaced with test compound dosing solution
- Incubate cells for 5 min at 37°C
- Terminate the incubation and lyse the cells
- Analyze cell lysate using LC-MS/MS to determine PAH concentration
- Determine protein concentration in cell lysate and normalize PAH concentration to protein content
Assay QC
- Uptake rate of probe substrate in OAT1-transfected vs. vector control cells
Notes
- The results from this assay are provided to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- The solubility of the test compound in Hanks’ buffer containing < 0.8% DMSO must be greater than the test concentration. If the solubility of the test compound is unavailable, Absorption Systems can conduct a solubility assessment at an additional charge.
- Percent inhibition is calculated as 100 x [1-(IROAT1-IRVC)TC / (IROAT1 – IRVC)0], where IROAT1 is the influx rate of PAH in OAT1-transfected cells, IRVC is the influx rate in vector control cells, “TC” means in the presence of test compound, and “0” means in the absence of test compound.
Options
The customer can request that a positive control be performed in parallel (additional fees apply)
This OAT3 transporter assay is used to screen for inhibition of OAT3 by a test compound in OAT3-transfected HEK293 cells at a single concentration
Required from Customer
- Either a minimum of 300 µL of test compound at 10 mM in DMSO, or 5 mg of powder
- Exact molecular mass of test compound and its salt form
- Relevant solubility of the test compound
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Normalized uptake of furosemide in the presence and absence of test compound in OAT3-transfected and vector control cells
- Percent inhibition by the test compound
- >50% inhibition indicates that the test compound is a significant inhibitor of OAT3
Substrate
- OAT3 probe substrate furosemide at 5 μM
- Test compound at 10 μM in modified Hanks’ buffer with less than 0.8% DMSO
Assay System
- OAT3-transfected HEK293 cells and vector control cells cultured in 24-well plates
Assay Conditions
- Measure uptake of furosemide in OAT3-transfected and vector control cells with and without test compound
- Single concentration of test compound
- Two cell lines: OAT3-transfected and vector control cell lines
- Four treatments as follows:
- OAT3-transfected cells with furosemide +/-test compound
- Vector control cells with furosemide +/- test compound
- Perform assay in duplicate (N=2 per treatment)
- Wells are equilibrated with blank buffer containing 1% BSA prior to initiating the assay
- Buffer is aspirated and replaced with test compound dosing solution
- Incubate cells for 5 min at 37°C
- Terminate the incubation and lyse the cells
- Analyze cell lysate using LC-MS/MS to determine furosemide concentration
- Determine protein concentration in cell lysate and normalize furosemide concentration to protein content
Assay QC
- Uptake rate of probe substrate in OAT3-transfected vs. vector control cells
Notes
- The results from this assay are provided to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- The solubility of the test compound in Hanks’ buffer containing < 0.8% DMSO must be greater than the test concentration. If the solubility of the test compound is unavailable, Absorption Systems can conduct a solubility assessment at an additional charge.
- Percent inhibition is calculated as 100 x [1-(IROAT3-IRVC)TC / (IROAT3 – IRVC)0], where IROAT3 is the influx rate of furosemide in OAT3-transfected cells, IRVCis the influx rate in vector control cells, “TC” means in the presence of test compound, and “0” means in the absence of test compound.
Options
The customer can request that a positive control be performed in parallel (additional fees apply)
This assay is used to screen for inhibition of OCT1 by a test compound in OCT1-transfected HEK293 cells at a single concentration.
Required from Customer
- Either a minimum of 300 µL of test compound at 10 mM in DMSO, or 5 mg of powder
- Exact molecular mass of test compound and its salt form
- Relevant solubility of the test compound
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Normalized uptake of MPP+ in the presence and absence of test compound in OCT1-transfected and vector control cells
- Percent inhibition by the test compound
- >50% inhibition indicates that the test compound is a significant inhibitor of OCT1
Substrate
- OCT1 probe substrate 1-methyl-4-Phenylpyridinium (MPP+) at 5 μM
- Test compound at 10 μM in modified Hanks’ buffer with less than 0.8% DMSO
Assay System
- OCT1-transfected HEK293 cells and vector control cells cultured in 24-well plates
Assay Conditions
- Measure uptake of MPP+ in OCT1-transfected and vector control cells with and without test compound
- Single concentration of test compound
- Two cell lines: OCT1-transfected and vector control cell lines
- Four treatments as follows:
- OCT1-transfected cells with MPP+ +/-test compound
- Vector control cells with MPP+ +/- test compound
- Perform assay in duplicate (N=2 per treatment)
- Wells are equilibrated with blank buffer containing 1% BSA prior to initiating the assay
- Buffer is aspirated and replaced with test compound dosing solution
- Incubate cells for 2 min at 37°C
- Terminate the incubation and lyse the cells
- Analyze cell lysate using LC-MS/MS to determine MPP+ concentration
- Determine protein concentration in cell lysate and normalize MPP+ concentration to protein content
Assay QC
- Uptake rate of probe substrate in OCT1-transfected vs. vector control cells
Notes
- The results from this assay are provided to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- The solubility of the test compound in Hanks’ buffer containing < 0.8% DMSO must be greater than the test concentration. If the solubility of the test compound is unavailable, Absorption Systems can conduct a solubility assessment at an additional charge.
- Percent inhibition is calculated as 100 x [1-(IROCT1-IRVC)TC / (IROCT1 – IRVC)0], where IROCT1 is the influx rate of MPP+ in OCT1-transfected cells, IRVC is the influx rate in vector control cells, “TC” means in the presence of test compound, and “0” means in the absence of test compound.
Options
The customer can request that a positive control be performed in parallel (additional fees apply)
This assay is used to screen for inhibition of OCT2 by a test compound in OCT2-transfected HEK293 cells at a single concentration.
Required from Customer
- Either a minimum of 300 µL of test compound at 10 mM in DMSO, or 5 mg of powder
- Exact molecular mass of test compound and its salt form
- Relevant solubility of the test compound
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Normalized uptake of MPP+ in the presence and absence of test compound in OCT2-transfected and vector control cells
- Percent inhibition by the test compound
- >50% inhibition indicates that the test compound is a significant inhibitor of OCT2
Substrate
- OCT2 probe substrate 1-methyl-4-Phenylpyridinium (MPP+) at 5 μM
- Test compound at 10 μM in modified Hanks’ buffer with less than 0.8% DMSO
Assay System
- OCT2-transfected HEK293 cells and vector control cells cultured in 24-well plates
Assay Conditions
- Measure uptake of MPP+ in OCT2-transfected and vector control cells with and without test compound
- Single concentration of test compound
- Two cell lines: OCT2-transfected and vector control cell lines
- Four treatments as follows:
- OCT2-transfected cells with MPP+ +/-test compound
- Vector control cells with MPP+ +/- test compound
- Perform assay in duplicate (N=2 per treatment)
- Wells are equilibrated with blank buffer containing 1% BSA prior to initiating the assay
- Buffer is aspirated and replaced with test compound dosing solution
- Incubate cells for 2 min at 37°C
- Terminate the incubation and lyse the cells
- Analyze cell lysate using LC-MS/MS to determine MPP+ concentration
- Determine protein concentration in cell lysate and normalize MPP+ concentration to protein content
Assay QC
- Uptake rate of probe substrate in OCT2-transfected vs. vector control cells
Notes
- The results from this assay are provided to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- The solubility of the test compound in Hanks’ buffer containing < 0.8% DMSO must be greater than the test concentration. If the solubility of the test compound is unavailable, Absorption Systems can conduct a solubility assessment at an additional charge.
- Percent inhibition is calculated as 100 x [1-(IROCT1-IRVC)TC / (IROCT1 – IRVC)0], where IROCT1 is the influx rate of MPP+ in OCT1-transfected cells, IRVC is the influx rate in vector control cells, “TC” means in the presence of test compound, and “0” means in the absence of test compound.
Options
The customer can request that a positive control be performed in parallel (additional fees apply)
This assay is used to screen for inhibition of OATP1B1 by a test compound in OATP1B1-transfected HEK293 cells at a single concentration
Required from Customer
- Either a minimum of 300 µL of test compound at 10 mM in DMSO, or 5 mg of powder
- Exact molecular mass of test compound and its salt form
- Relevant solubility of the test compound
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Normalized uptake of atorvastatin in the presence and absence of test compound in OATP1B1-transfected and vector control cells
- Percent inhibition by the test compound
- >50% inhibition indicates that the test compound is a significant inhibitor of OATP1B1
Substrate
- OATP1B1 probe substrate Atorvastatin at 0.15 μM
- Test compound at 10 μM in modified Hanks’ buffer with less than 0.8% DMSO
Assay System
- OATP1B1-transfected HEK293 cells and vector control cells cultured in 24-well plates
Assay Conditions
- Measure uptake of atorvastatin in OATP1B1-transfected and vector control cells with and without test compound
- Single concentration of test compound
- Two cell lines: OATP1B1-transfected and vector control cell lines
- Four treatments as follows:
- OATP1B1-transfected cells with atorvastatin +/-test compound
- Vector control cells with atorvastatin +/- test compound
- Perform assay in duplicate (N=2 per treatment)
- Wells are equilibrated with blank buffer containing 1% BSA prior to initiating the assay
- Buffer is aspirated and replaced with test compound dosing solution
- Incubate cells for 5 min at 37°C
- Terminate the incubation and lyse the cells
- Analyze cell lysate using LC-MS/MS to determine atorvastatin concentration
- Determine protein concentration in cell lysate and normalize atorvastatin concentration to protein content
Assay QC
- Uptake rate of probe substrate in OATP1B1-transfected vs. vector control cells
Notes
- The results from this assay are provided to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- The solubility of the test compound in Hanks’ buffer containing < 0.8% DMSO must be greater than the test concentration. If the solubility of the test compound is unavailable, Absorption Systems can conduct a solubility assessment at an additional charge.
- Percent inhibition is calculated as 100 x [1-(IR1B1-IRVC)TC / (IR1B1 – IRVC)0], where IR1B1 is the influx rate of atorvastatin in OATP1B1-transfected cells, IRVC is the influx rate in vector control cells, “TC” means in the presence of test compound, and “0” means in the absence of test compound.
Options
The customer can request that a positive control be performed in parallel (additional fees apply)
This OATP1B3 transporter assay is used to screen for inhibition of OATP1B3 by a test compound in OATP1B3-transfected HEK293 cells at a single concentration.
Required from Customer
- Either a minimum of 300 µL of test compound at 10 mM in DMSO, or 5 mg of powder
- Exact molecular mass of test compound and its salt form
- Relevant solubility of the test compound
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Normalized uptake of atorvastatin in the presence and absence of test compound in OATP1B3-transfected and vector control cells
- Percent inhibition by the test compound
- >50% inhibition indicates that the test compound is a significant inhibitor of OATP1B3
Substrate
- OATP1B3 probe substrate Atorvastatin at 0.15 μM
- Test compound at 10 μM in modified Hanks’ buffer with less than 0.8% DMSO
Assay System
- OATP1B3-transfected HEK293 cells and vector control cells cultured in 24-well plates
Assay Conditions
- Measure uptake of atorvastatin in OATP1B3-transfected and vector control cells with and without test compound
- Single concentration of test compound
- Two cell lines: OATP1B3-transfected and vector control cell lines
- Four treatments as follows:
- OATP1B3-transfected cells with atorvastatin +/-test compound
- Vector control cells with atorvastatin +/- test compound
- Perform assay in duplicate (N=2 per treatment)
- Wells are equilibrated with blank buffer containing 1% BSA prior to initiating the assay
- Buffer is aspirated and replaced with test compound dosing solution
- Incubate cells for 5 min at 37°C
- Terminate the incubation and lyse the cells
- Analyze cell lysate using LC-MS/MS to determine atorvastatin concentration
- Determine protein concentration in cell lysate and normalize atorvastatin concentration to protein content
Assay QC
- Uptake rate of probe substrate in OATP1B3-transfected vs. vector control cells
Notes
- The results from this assay are provided to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- The solubility of the test compound in Hanks’ buffer containing < 0.8% DMSO must be greater than the test concentration. If the solubility of the test compound is unavailable, Absorption Systems can conduct a solubility assessment at an additional charge.
- Percent inhibition is calculated as 100 x [1-(IR1B3-IRVC)TC / (IR1B3 – IRVC)0], where IR1B3 is the influx rate of atorvastatin in OATP1B3-transfected cells, IRVC is the influx rate in vector control cells, “TC” means in the presence of test compound, and “0” means in the absence of test compound.
Options
The customer can request that a positive control be performed in parallel (additional fees apply)
This assay is used to determine the IC50 of a test compound for inhibition of P-gp in MDR1-MDCK cells
Required from Customer
- Either a minimum of 300 µL of test compound at 20 mM in DMSO, or 10 mg of powder
- Exact molecular mass of test compound and its salt form
- Solubility and chemical stability of the test compound in Hanks’ buffer
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Apparent permeability (Papp) of digoxin in the basolateral-to-apical direction, in the presence and absence of test compound
- IC50 of the test compound
Substrate
- P-gp probe substrate digoxin at 10 µM
- Test compound at six concentrations (serial diluations from 100 µM) in modified Hanks’ buffer with less than 0.8% DMSO
Assay System
- Confluent monolayers of MDR1-MDCK cells, 7-14 days old, in Transwell® dual-chamber plates, with apical and basolateral pH 7.4
Assay Conditions
- Monolayers pretreated and co-dosed with test compound on both apical and basolateral sides
- Six concentrations of test compound
- Single incubation (n=1) per concentration
- Negative control: vehicle only
- Duplicate incubations (n=2)
- Positive control: single concentration of known inhibitor Valspodar
- Duplicate incubations (n=2)
- Dose digoxin on basolateral side only for B to A permeability
- Sample both apical and basolateral sides at 120 minutes
- Determine the concentration of digoxin using a generic LC-MS/MS method with a minimum 4-point calibration curve
Assay QC
- Verify the quality of the cell monolayer batch using control compounds before the monolayers are released for use
- Verify the quality of each monolayer used in an assay by a pre-experiment TEER measurement and by calculating the Papp for the co-dosed control compound, Lucifer yellow
Notes
- The results from this assay are provided to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- The solubility of the test compound in Hanks’ buffer containing < 0.8% DMSO must be greater than the test concentration. If the solubility of the test compound is unavailable, Absorption Systems can conduct a solubility assessment at an additional charge.
This P-gp transporter assay is used to determine the IC50 of a test compound for inhibition of P-gp in Caco-2 cells.
Required from Customer
- Either a minimum of 300 µL of test compound at 20 mM in DMSO, or 10 mg of powder
- Exact molecular mass of test compound and its salt form
- Solubility and chemical stability of the test compound in Hanks’ buffer
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Apparent permeability (Papp) of digoxin in the basolateral-to-apical direction, in the presence and absence of test compound
- IC50 of the test compound
Substrate
- P-gp probe substrate digoxin at 10 µM
- Test compound at six concentrations (serial diluations from 100 µM) in modified Hanks’ buffer with less than 0.8% DMSO
Assay System
- Confluent monolayers of Caco-2 cells, 21-28 days old, in Transwell® dual-chamber plates, with apical and basolateral pH 7.4
Assay Conditions
- Monolayers pretreated and co-dosed with test compound on both apical and basolateral sides
- Six concentrations of test compound
- Single incubation (n=1) per concentration
- Negative control: vehicle only
- Duplicate incubations (n=2)
- Positive control: single concentration of known inhibitor Valspodar
- Duplicate incubations (n=2)
- Dose digoxin on basolateral side only for B to A permeability
- Sample both apical and basolateral sides at 120 minutes
- Determine the concentration of digoxin using a generic LC-MS/MS method with a minimum 4-point calibration curve
Assay QC
- Verify the quality of the cell monolayer batch using control compounds before the monolayers are released for use
- Verify the quality of each monolayer used in an assay by a pre-experiment TEER measurement and by calculating the Papp for the co-dosed control compound, Lucifer yellow
Notes
- The results from this assay are provided to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- The solubility of the test compound in Hanks’ buffer containing < 0.8% DMSO must be greater than the test concentration. If the solubility of the test compound is unavailable, Absorption Systems can conduct a solubility assessment at an additional charge.
This assay is used to determine the IC50 of a test compound for inhibition of BCRP in BCRP-MDCK cells.
Required from Customer
- Either a minimum of 300 µL of test compound at 20 mM in DMSO, or 10 mg of powder
- Exact molecular mass of test compound and its salt form
- Solubility and chemical stability of the test compound in Hanks’ buffer
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Apparent permeability (Papp) of cladribine in the basolateral-to-apical direction, in the presence and absence of test compound
- IC50 of the test compound
Substrate
- BCRP probe substrate cladribine at 10 µM
- Test compound at six concentrations (serial dilutions from 100µM) in modified Hanks’ buffer with less than 0.8% DMSO
Assay System
- Confluent monolayers of BCRP-MDCK cells, 7-14 days old, in Transwell® dual-chamber plates, with apical and basolateral pH 7.4
Assay Conditions
- Monolayers pretreated and co-dosed with test compound on both apical and basolateral sides
- Six concentrations of test compound
- Single incubation (n=1) per concentration
- Negative control: vehicle only
- Duplicate incubations (n=2)
- Positive control: single concentration of known inhibitor Ko143
- Duplicate incubations (n=2)
- Dose cladribine on basolateral side only for B to A permeability
- Sample both apical and basolateral sides at 120 minutes
- Determine the concentration of cladribine using a generic LC-MS/MS method with a minimum 4-point calibration curve
Assay QC
- Verify the quality of the cell monolayer batch using control compounds before the monolayers are released for use
- Verify the quality of each monolayer used in an assay by a pre-experiment TEER measurement and by calculating the Papp for the co-dosed control compound, Lucifer yellow
Notes
- The results from this assay are provided to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- The solubility of the test compound in Hanks’ buffer containing < 0.8% DMSO must be greater than the test concentration. If the solubility of the test compound is unavailable, Absorption Systems can conduct a solubility assessment at an additional charge.
This assay is used to determine the IC50 of a test compound for inhibition of OAT1 in OAT1-transfected HEK293 cells
Required from Customer
- Either a minimum of 300 µL of test compound at 20 mM in DMSO, or 10 mg of powder
- Exact molecular mass of test compound and its salt form
- Relevant solubility of the test compound
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Normalized uptake of para-aminohippurate (PAH) in the presence and absence of test compound in OAT1-transfected and vector control cells
- IC50 of the test compound
Substrate
- OAT1 probe substrate PAH at 10 μM
- Test compound at six concentrations (serial diluations from 100 µM) in modified Hanks’ buffer with less than 0.8% DMSO
Assay System
- OAT1-transfected HEK293 cells and vector control cells cultured in 24-well plates
Assay Conditions
- Measure uptake of PAH in OAT1-transfected and vector control cells with and without test compound
- Six concentrations of test compound
- Single incubation (n=1) per concentration
- Negative control: vehicle only
- Duplicate incubations (n=2)
- Positive control: single concentration of known inhibitor probenecid
- Duplicate incubations (n=2)
- Two cell lines: OAT1-transfected and vector control cell lines
- Treatments performed in each cell line:
- Wells are equilibrated with blank buffer containing 1% BSA prior to initiating the assay
- Buffer is aspirated and replaced with dosing solution
- Incubate cells for 5 min at 37°C
- Terminate the incubation and lyse the cells
- Analyze cell lysate using LC-MS/MS to determine PAH concentration
- Determine protein concentration in cell lysate and normalize PAH concentration to protein content
Assay QC
- Uptake rate of probe substrate in OAT1-transfected vs. vector control cells
- Inhibition of probe substrate uptake by a known inhibitor of OAT1
Notes
- The results from this assay are provided to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- The solubility of the test compound in Hanks’ buffer containing < 0.8% DMSO must be greater than the test concentration. If the solubility of the test compound is unavailable, Absorption Systems can conduct a solubility assessment at an additional charge.
This assay is used to determine the IC50 of a test compound for inhibition of OAT3 in OAT3-transfected HEK293 cells.
Required from Customer
- Either a minimum of 300 µL of test compound at 20 mM in DMSO, or 10 mg of powder
- Exact molecular mass of test compound and its salt form
- Relevant solubility of the test compound
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Normalized uptake of furosemide in the presence and absence of test compound in OAT3-transfected and vector control cells
- IC50 of the test compound
Substrate
- OAT3 probe substrate PAH at 10 μM
- Test compound at six concentrations (serial diluations from 100 µM) in modified Hanks’ buffer with less than 0.8% DMSO
Assay System
- OAT3-transfected HEK293 cells and vector control cells cultured in 24-well plates
Assay Conditions
- Measure uptake of PAH in OAT3-transfected and vector control cells with and without test compound
- Six concentrations of test compound
- Single incubation (n=1) per concentration
- Negative control: vehicle only
- Duplicate incubations (n=2)
- Positive control: single concentration of known inhibitor probenecid
- Duplicate incubations (n=2)
- Two cell lines: OAT3-transfected and vector control cell lines
- Treatments performed in each cell line:
- Wells are equilibrated with blank buffer containing 1% BSA prior to initiating the assay
- Buffer is aspirated and replaced with dosing solution
- Incubate cells for 5 min at 37°C
- Terminate the incubation and lyse the cells
- Analyze cell lysate using LC-MS/MS to determine PAH concentration
- Determine protein concentration in cell lysate and normalize PAH concentration to protein content
Assay QC
- Uptake rate of probe substrate in OAT3-transfected vs. vector control cells
- Inhibition of probe substrate uptake by a known inhibitor of OAT3
Notes
- The results from this assay are provided to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- The solubility of the test compound in Hanks’ buffer containing < 0.8% DMSO must be greater than the test concentration. If the solubility of the test compound is unavailable, Absorption Systems can conduct a solubility assessment at an additional charge.
This assay is used to determine the IC50 of a test compound for inhibition of OCT1 in OCT1-transfected HEK293 cells.
Required from Customer
- Either a minimum of 300 µL of test compound at 20 mM in DMSO, or 10 mg of powder
- Exact molecular mass of test compound and its salt form
- Relevant solubility of the test compound
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Normalized uptake of MPP+ in the presence and absence of test compound in OCT2-transfected and vector control cells
- IC50 of the test compound
Substrate
- OCT2 probe substrate 1-methyl-4-Phenylpyridinium (MPP+) at 5 μM
- Test compound at six concentrations (serial diluations from 100 µM) in modified Hanks’ buffer with less than 0.8% DMSO
Assay System
- OCT2-transfected HEK293 cells and vector control cells cultured in 24-well plates
Assay Conditions
- Measure uptake of MPP+ in OCT1-transfected and vector control cells with and without test compound
- Six concentrations of test compound
- Single incubation (n=1) per concentration
- Negative control: vehicle only
- Duplicate incubations (n=2)
- Positive control: single concentration of known inhibitor repaglinide
- Duplicate incubations (n=2)
- Two cell lines: OCT1-transfected and vector control cell lines
- Treatments performed in each cell line:
- Wells are equilibrated with blank buffer containing 1% BSA prior to initiating the assay
- Buffer is aspirated and replaced with dosing solution
- Incubate cells for 2 min at 37°C
- Terminate the incubation and lyse the cells
- Analyze cell lysate using LC-MS/MS to determine MPP+ concentration
- Determine protein concentration in cell lysate and normalize MPP+ concentration to protein content
Assay QC
- Uptake rate of probe substrate in OCT2-transfected vs. vector control cells
- Inhibition of probe substrate uptake by a known inhibitor of OCT2
Notes
- The results from this assay are provided to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- The solubility of the test compound in Hanks’ buffer containing < 0.8% DMSO must be greater than the test concentration. If the solubility of the test compound is unavailable, Absorption Systems can conduct a solubility assessment at an additional charge.
This OCT transporter assay is used to determine the IC50 of a test compound for inhibition of OCT2 in OCT2-transfected HEK293 cells.
Required from Customer
- Either a minimum of 300 µL of test compound at 20 mM in DMSO, or 10 mg of powder
- Exact molecular mass of test compound and its salt form
- Relevant solubility of the test compound
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Normalized uptake of MPP+ in the presence and absence of test compound in OCT2-transfected and vector control cells
- IC50 of the test compound
Substrate
- OCT2 probe substrate 1-methyl-4-Phenylpyridinium (MPP+) at 5 μM
- Test compound at six concentrations (serial diluations from 100 µM) in modified Hanks’ buffer with less than 0.8% DMSO
Assay System
- OCT2-transfected HEK293 cells and vector control cells cultured in 24-well plates
Assay Conditions
- Measure uptake of MPP+ in OCT1-transfected and vector control cells with and without test compound
- Six concentrations of test compound
- Single incubation (n=1) per concentration
- Negative control: vehicle only
- Duplicate incubations (n=2)
- Positive control: single concentration of known inhibitor repaglinide
- Duplicate incubations (n=2)
- Two cell lines: OCT1-transfected and vector control cell lines
- Treatments performed in each cell line:
- Wells are equilibrated with blank buffer containing 1% BSA prior to initiating the assay
- Buffer is aspirated and replaced with dosing solution
- Incubate cells for 2 min at 37°C
- Terminate the incubation and lyse the cells
- Analyze cell lysate using LC-MS/MS to determine MPP+ concentration
- Determine protein concentration in cell lysate and normalize MPP+ concentration to protein content
Assay QC
- Uptake rate of probe substrate in OCT2-transfected vs. vector control cells
- Inhibition of probe substrate uptake by a known inhibitor of OCT2
Notes
- The results from this assay are provided to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- The solubility of the test compound in Hanks’ buffer containing < 0.8% DMSO must be greater than the test concentration. If the solubility of the test compound is unavailable, Absorption Systems can conduct a solubility assessment at an additional charge.
This assay is used to determine the IC50 of a test compound for inhibition of OATP1B1 in OATP1B1-transfected HEK293 cells.
Required from Customer
- Either a minimum of 300 µL of test compound at 20 mM in DMSO, or 10 mg of powder
- Exact molecular mass of test compound and its salt form
- Relevant solubility of the test compound
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Normalized uptake of atorvastatin in the presence and absence of test compound in OATP1B1-transfected and vector control cells
- IC50 of the test compound
Substrate
- OATP1B1 probe substrate atorvastatin at 0.15 μM
- Test compound at six concentrations (serial diluations from 100 µM) in modified Hanks’ buffer with less than 0.8% DMSO
Assay System
- OATP1B1-transfected HEK293 cells and vector control cells cultured in 24-well plates
Assay Conditions
- Measure uptake of atorvastatin in OATP1B1-transfected and vector control cells with and without test compound
- Six concentrations of test compound
- Single incubation (n=1) per concentration
- Negative control: vehicle only
- Duplicate incubations (n=2)
- Positive control: single concentration of known inhibitor rifamycin SV
- Duplicate incubations (n=2)
- Two cell lines: OATP1B1-transfected and vector control cell lines
- Treatments performed in each cell line:
- Wells are equilibrated with blank buffer containing 1% BSA prior to initiating the assay
- Buffer is aspirated and replaced with dosing solution
- Incubate cells for 5 min at 37°C
- Terminate the incubation and lyse the cells
- Analyze cell lysate using LC-MS/MS to determine atorvastatin concentration
- Determine protein concentration in cell lysate and normalize atorvastatin concentration to protein content
Assay QC
- Uptake rate of probe substrate in OATP1B1-transfected vs. vector control cells
- Inhibition of probe substrate uptake by a known inhibitor of OATP1B1
Notes
- The results from this assay are provided to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- The solubility of the test compound in Hanks’ buffer containing < 0.8% DMSO must be greater than the test concentration. If the solubility of the test compound is unavailable, Absorption Systems can conduct a solubility assessment at an additional charge.
This assay is used to determine the IC50 of a test compound for inhibition of OATP1B3 in OATP1B3-transfected HEK293 cells.
Required from Customer
- Either a minimum of 300 µL of test compound at 20 mM in DMSO, or 10 mg of powder
- Exact molecular mass of test compound and its salt form
- Relevant solubility of the test compound
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Normalized uptake of atorvastatin in the presence and absence of test compound in OATP1B3-transfected and vector control cells
- IC50 of the test compound
Substrate
- OATP1B3 probe substrate atorvastatin at 0.15 μM
- Test compound at six concentrations (serial diluations from 100 µM) in modified Hanks’ buffer with less than 0.8% DMSO
Assay System
- OATP1B3-transfected HEK293 cells and vector control cells cultured in 24-well plates
Assay Conditions
- Measure uptake of atorvastatin in OATP1B3-transfected and vector control cells with and without test compound
- Six concentrations of test compound
- Single incubation (n=1) per concentration
- Negative control: vehicle only
- Duplicate incubations (n=2)
- Positive control: single concentration of known inhibitor rifamycin SV
- Duplicate incubations (n=2)
- Two cell lines: OATP1B3-transfected and vector control cell lines
- Treatments performed in each cell line:
- Wells are equilibrated with blank buffer containing 1% BSA prior to initiating the assay
- Buffer is aspirated and replaced with dosing solution
- Incubate cells for 5 min at 37°C
- Terminate the incubation and lyse the cells
- Analyze cell lysate using LC-MS/MS to determine atorvastatin concentration
- Determine protein concentration in cell lysate and normalize atorvastatin concentration to protein content
Assay QC
- Uptake rate of probe substrate in OATP1B3-transfected vs. vector control cells
- Inhibition of probe substrate uptake by a known inhibitor of OATP1B3
Notes
- The results from this assay are provided to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- The solubility of the test compound in Hanks’ buffer containing < 0.8% DMSO must be greater than the test concentration. If the solubility of the test compound is unavailable, Absorption Systems can conduct a solubility assessment at an additional charge.
This BSEP transporter assay is used to screen for inhibition of BSEP by a test compound in hBSEP-expressing vesicles at a single concentration
Required from Customer
- Either a minimum of 300 µL of test compound at 10 mM in DMSO, or 5 mg of powder
- Exact molecular mass of test compound and its salt form
- Relevant solubility of the test compound
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Normalized uptake of 3H-taurocholic acid in the presence and absence of test compound in hBSEP-expressing vesicles
- Percent inhibition by the test compound
- >50% inhibition indicates that the test compound is a significant inhibitor of BSEP
Substrate
- BSEP probe substrate 3H-taurocholic acid at 1 μM
- Test compound at 10 μM in Hepes Influx Buffer
Assay System
- hBSEP-expressing vesicles in the presence of ATP vs. AMP
Assay Conditions
- Measure uptake of 3H-taurocholic acid in hBSEP-expressing vesicles with and without test compound, in the presence of ATP vs. AMP
- Single concentration of test compound
- Four treatments as follows:
- hBSEP-expressing vesicles cells with 3H-taurocholic acid +/-test compound, in the presence of ATP and AMP (separately)
- Perform assay in duplicate (N=2 per treatment)
- Incubate cells for 30 min at 37°C
- Terminate the incubation
- Measure radioactivity of processed samples to determine 3H-taurocholic acid concentration
Assay QC
- Uptake rate of probe substrate in hBSEP-expressing vesicles in the presence of ATP vs. AMP
Notes
- The results from this assay are provided to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- The solubility of the test compound in Hepes Influx Buffer must be greater than the test concentration. If the solubility of the test compound is unavailable, Absorption Systems can conduct a solubility assessment at an additional charge.
- Percent inhibition is calculated as 100 x [1-(IRATP-IRAMP)TC / (IRATP – IRAMP)0], where IRATP is the influx rate of 3H-taurocholic acid in hBSEP-expressing vesicles in the presence of ATP, IRAMP is the influx rate of 3H-taurocholic acid in hBSEP-expressing vesicles in the presence of AMP, “TC” means in the presence of test compound, and “0” means in the absence of test compound.
Options
The customer can request: that a positive control be performed in parallel (additional fees apply)
This assay is used to determine the IC50 of a test compound for inhibition of BSEP in hBSEP-expressing vesicles.
Required from Customer
- Either a minimum of 300 µL of test compound at 10 mM in DMSO, or 10 mg of powder
- Exact molecular mass of test compound and its salt form
- Solubility of test compound in Hanks’ buffer
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Normalized uptake of 3H-taurocholic acid in the presence and absence of test compound in hBSEP-expressing vesicles
- IC50 of the test compound
Substrate
- BSEP probe substrate 3H-taurocholic acid at 1 μM
- Test compound at six concentrations (serial diluations from 100 µM) in Hepes Influx Buffer
Assay System
- hBSEP-expressing vesicles in the presence of ATP vs. AMP
Assay Conditions
- Measure uptake of 3H-taurocholic acid in hBSEP-expressing vesicles with and without test compound, in the presence of ATP vs. AMP
- Six concentrations of test compound
- Single incubation (n=1) per concentration
- Negative control: vehicle only
- Duplicate incubations (n=2)
- Positive control: single concentration of known inhibitor CsA
- Duplicate incubations (n=2)
- Treatments performed in in the presence of ATP and AMP (separately):
- Incubate cells for 30 min at 37°C
- Terminate the incubation
- Measure radioactivity of processed samples to determine 3H-taurocholic acid concentration
Assay QC
- Uptake rate of probe substrate in hBSEP-expressing vesicles in the presence of ATP vs. AMP
- Inhibition of probe substrate uptake by a known inhibitor of BSEP
Notes
- The results from this assay are provided to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- The solubility of the test compound in Hepes Influx Buffer must be greater than the test concentration. If the solubility of the test compound is unavailable, Absorption Systems can conduct a solubility assessment at an additional charge.
This assay is used to screen for inhibition of MATE1 by a test compound in MATE1-transfected HEK293 cells at a single concentration.
Required from Customer
- Either a minimum of 300 µL of test compound at 10 mM in DMSO, or 5 mg of powder
- Exact molecular mass of test compound and its salt form
- Relevant solubility of the test compound
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Normalized uptake of Metformin in the presence and absence of test compound in MATE1-transfected and vector control cells
- Percent inhibition by the test compound
- >50% inhibition indicates that the test compound is a significant inhibitor of MATE1
Substrate
- MATE1 probe substrate Metformin at 50 μM
- Test compound at 10 μM in modified Hanks’ buffer with less than 0.8% DMSO
Assay System
- MATE1-transfected HEK293 cells and vector control cells cultured in 24-well plates
Assay Conditions
- Measure uptake of Metformin in MATE1-transfected and vector control cells with and without test compound
- Single concentration of test compound
- Two cell lines: MATE1-transfected and vector control cell lines
- Four treatments as follows:
- MATE1-transfected cells with Metformin +/-test compound
- Vector control cells with Metformin +/- test compound
- Perform assay in duplicate (N=2 per treatment)
- Wells are equilibrated with blank buffer containing 1% BSA prior to initiating the assay
- Buffer is aspirated and replaced with test compound dosing solution
- Incubate cells for 5 min at 37°C
- Terminate the incubation and lyse the cells
- Analyze cell lysate using LC-MS/MS to determine Metformin concentration
- Determine protein concentration in cell lysate and normalize Metformin concentration to protein content
Assay QC
- Uptake rate of probe substrate in MATE1-transfected vs. vector control cells
Notes
- The results from this assay are provided to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- The solubility of the test compound in Hanks’ buffer containing < 0.8% DMSO must be greater than the test concentration. If the solubility of the test compound is unavailable, Absorption Systems can conduct a solubility assessment at an additional charge.
- Percent inhibition is calculated as 100 x [1-(IR1B1-IRVC)TC / (IR1B1 – IRVC)0], where IR1B1 is the influx rate of Metformin in MATE1-transfected cells, IRVC is the influx rate in vector control cells, “TC” means in the presence of test compound, and “0” means in the absence of test compound.
Options
The customer can request: that a positive control be performed in parallel (additional fees apply)
This assay is used to screen for inhibition of MATE2K by a test compound in MATE2K-transfected HEK293 cells at a single concentration
Required from Customer
- Either a minimum of 300 µL of test compound at 10 mM in DMSO, or 5 mg of powder
- Exact molecular mass of test compound and its salt form
- Relevant solubility of the test compound
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Normalized uptake of Metformin in the presence and absence of test compound in MATE2K-transfected and vector control cells
- Percent inhibition by the test compound
- >50% inhibition indicates that the test compound is a significant inhibitor of MATE2K
Substrate
- MATE2K probe substrate Metformin at 50 μM
- Test compound at 10 μM in modified Hanks’ buffer with less than 0.8% DMSO
Assay System
- MATE2K-transfected HEK293 cells and vector control cells cultured in 24-well plates
Assay Conditions
- Measure uptake of Metformin in MATE2K-transfected and vector control cells with and without test compound
- Single concentration of test compound
- Two cell lines: MATE2K-transfected and vector control cell lines
- Four treatments as follows:
- MATE2K-transfected cells with Metformin +/-test compound
- Vector control cells with Metformin +/- test compound
- Perform assay in duplicate (N=2 per treatment)
- Wells are equilibrated with blank buffer containing 1% BSA prior to initiating the assay
- Buffer is aspirated and replaced with test compound dosing solution
- Incubate cells for 5 min at 37°C
- Terminate the incubation and lyse the cells
- Analyze cell lysate using LC-MS/MS to determine Metformin concentration
- Determine protein concentration in cell lysate and normalize Metformin concentration to protein content
Assay QC
- Uptake rate of probe substrate in MATE2K-transfected vs. vector control cells
Notes
- The results from this assay are provided to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- The solubility of the test compound in Hanks’ buffer containing < 0.8% DMSO must be greater than the test concentration. If the solubility of the test compound is unavailable, Absorption Systems can conduct a solubility assessment at an additional charge.
- Percent inhibition is calculated as 100 x [1-(IR1B1-IRVC)TC / (IR1B1 – IRVC)0], where IR1B1 is the influx rate of Metformin in MATE2K-transfected cells, IRVC is the influx rate in vector control cells, “TC” means in the presence of test compound, and “0” means in the absence of test compound.
Options
The customer can request: that a positive control be performed in parallel (additional fees apply)
This assay is used to determine the IC50 of a test compound for inhibition of MATE1 in MATE1-transfected HEK293 cells.
Required from Customer
- Either a minimum of 300 µL of test compound at 20 mM in DMSO, or 10 mg of powder
- Exact molecular mass of test compound and its salt form
- Relevant solubility of the test compound
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Normalized uptake of Metformin in the presence and absence of test compound in MATE1-transfected and vector control cells
- IC50 of the test compound
Substrate
- MATE1 probe substrate Metformin at 50 μM
- Test compound at six concentrations (serial diluations from 100 µM) in modified Hanks’ buffer with less than 0.8% DMSO
Assay System
- MATE1-transfected HEK293 cells and vector control cells cultured in 24-well plates
Assay Conditions
- Measure uptake of Metformin in MATE1-transfected and vector control cells with and without test compound
- Six concentrations of test compound
- Single incubation (n=1) per concentration
- Negative control: vehicle only
- Duplicate incubations (n=2)
- Positive control: single concentration of known inhibitor Cimetidine
- Duplicate incubations (n=2)
- Two cell lines: MATE1-transfected and vector control cell lines
- Treatments performed in each cell line:
- Wells are equilibrated with blank buffer containing 1% BSA prior to initiating the assay
- Buffer is aspirated and replaced with dosing solution
- Incubate cells for 5 min at 37°C
- Terminate the incubation and lyse the cells
- Analyze cell lysate using LC-MS/MS to determine Metformin concentration
- Determine protein concentration in cell lysate and normalize Metformin concentration to protein content
Assay QC
- Uptake rate of probe substrate in MATE1-transfected vs. vector control cells
- Inhibition of probe substrate uptake by a known inhibitor of MATE1
Notes
- The results from this assay are provided to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- The solubility of the test compound in Hanks’ buffer containing < 0.8% DMSO must be greater than the test concentration. If the solubility of the test compound is unavailable, Absorption Systems can conduct a solubility assessment at an additional charge.
This assay is used to determine the IC50 of a test compound for inhibition of MATE2K in MATE2K-transfected HEK293 cells.
Required from Customer
- Either a minimum of 300 µL of test compound at 20 mM in DMSO, or 10 mg of powder
- Exact molecular mass of test compound and its salt form
- Relevant solubility of the test compound
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Normalized uptake of Metformin in the presence and absence of test compound in MATE2K-transfected and vector control cells
- IC50 of the test compound
Substrate
- MATE2K probe substrate Metformin at 50 μM
- Test compound at six concentrations (serial diluations from 100 µM) in modified Hanks’ buffer with less than 0.8% DMSO
Assay System
- MATE2K-transfected HEK293 cells and vector control cells cultured in 24-well plates
Assay Conditions
- Measure uptake of Metformin in MATE2K-transfected and vector control cells with and without test compound
- Six concentrations of test compound
- Single incubation (n=1) per concentration
- Negative control: vehicle only
- Duplicate incubations (n=2)
- Positive control: single concentration of known inhibitor Pyrimethamine
- Duplicate incubations (n=2)
- Two cell lines: MATE2K-transfected and vector control cell lines
- Treatments performed in each cell line:
- Wells are equilibrated with blank buffer containing 1% BSA prior to initiating the assay
- Buffer is aspirated and replaced with dosing solution
- Incubate cells for 5 min at 37°C
- Terminate the incubation and lyse the cells
- Analyze cell lysate using LC-MS/MS to determine Metformin concentration
- Determine protein concentration in cell lysate and normalize Metformin concentration to protein content
Assay QC
- Uptake rate of probe substrate in MATE2K-transfected vs. vector control cells
- Inhibition of probe substrate uptake by a known inhibitor of MATE2K
Notes
- The results from this assay are provided to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- The solubility of the test compound in Hanks’ buffer containing < 0.8% DMSO must be greater than the test concentration. If the solubility of the test compound is unavailable, Absorption Systems can conduct a solubility assessment at an additional charge.
Objective
To determine the IC50 of the Customer’s test article towards BCRP in Caco-2 cell monolayers
Required from Customer
- A minimum of 100 µL of test article at 100 mM in DMSO stock solution or 5 mg powder
- Molecular weight of the test article and its salt form
- MSDS or handling and storage information
Deliverables
Express Plus containing:
- Materials and methods
- Results:
- The apparent permeability (Papp) of cladribine in the presence and absence of test article and/or known inhibitor
- The IC50 of the test article towards BCRP
- Appendix including results of cell batch certification
Substrate
- Cladribine and test article in HBSSg with maximum DMSO concentration ≤ 0.8%
Test System
- Twenty-one (21) to twenty-eight (28) days-in-culture Caco-2 cells plated in Transwell™ dual chamber plates
- Each batch of Caco-2 cell monolayers will be certified using internally established criteria
Assay Conditions
- Unidirectional permeability assessment of cladribine in cultured Caco-2 cells with and without test article
- Transport buffer: HBSSg, pH 7.4 ± 0.2
- Treatments:
| Treatment | Permeability | Replicates | Test article Concentration | Cladribine Concentration | Donor and Receiver Sampling Time Point |
|---|---|---|---|---|---|
| With Test article | B➞A | 2 | 3-fold serial dilutions from 100 µM (6 concentrations) | 10 µM | 120 min |
| Without Test article | 2 | N/A | |||
| With Ko143 (0.5 µM) | 2 | N/A |
NOTE: Cells treated with inhibitors will be pre-incubated with inhibitor for 30 minutes prior to assay onset. Cells used for testing with no inhibitors will be pre-incubated with blank Hanks buffer for 30 minutes prior to assay onset.
- LC/MS/MS analysis of cladribine with a minimum 6-point standard curve
- At least 60% standards must be accurate to within ±20%, except at the Lower Limit of Quantitation (LLOQ), where ±25% will be considered acceptable
- Monolayer integrity confirmed via post-experiment Lucifer Yellow permeability assessment
Assay QC
- The quality of the monolayer batch is verified using control compounds before the monolayers are released for use
- The quality of each monolayer used in the assay is verified by calculating the Papp for the control compound lucifer yellow post-experiment
Objective
To determine the bidirectional permeability of cladribine across Caco-2 cell monolayers in the absence and presence of test article, in order to assess if the Sponsor’s test article is a possible BCRP inhibitor
Required from Customer
- A minimum of 100 µL of test article at 10 mM in DMSO stock solution or 1 mg powder
- Molecular weight of the test article and its salt form
- MSDS or handling and storage information
Deliverables
Express Plus containing:
- Materials and methods
- Results:
- The apparent permeability (Papp) of cladribine in both directions
Papp = (dCr /dt) x Vr/(A x CA)
Where,
dCr /dt is the slope of the cumulative concentration in the receiver compartment versus time in µM s-1;
Vr is the volume of the receiver compartment in cm3;
A is the area of the insert (1.13 cm2 for 12-well Transwell®);
CA is the average of the nominal dosing concentration and the measured 120 minute donor concentration in µM - The efflux ratio (ER)
ER =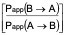
- The BCRP inhibitor potential of a test article classified as either positive or negative. If the corrected efflux ratio of cladribine (CERC) is reduced by more than 50% in the presence of the test article (CERC/TC), then the test article is classified as a BCRP inhibitor.
CERC = ER-1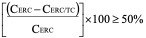
- The apparent permeability (Papp) of cladribine in both directions
- Appendix including:
- Analytical method
- Results of batch certification
Substrate
Cladribine and test article each at 10 µM in HBSSg with DMSO concentration ≤ 0.8%
Test System
- Twenty-one (21) to twenty-eight (28) days-in-culture Caco-2 cells plated in Transwell™ dual chamber plates
- Each batch of Caco-2 cell monolayers will be certified using internally established criteria
Assay Conditions
- Bidirectional permeability assessment of cladribine in cultured Caco-2 cells with and without test article
- Transport buffer: HBSSg, pH 7.4 ± 0.2
- Test article treatments:
| Treatment | Permeability | Replicates | Test article Concentration | Cladribine Concentration | Donor and Receiver Sampling Time Point |
|---|---|---|---|---|---|
| With Test article | Bidirectional | 2 | 10 µM | 10 µM | 120 min |
| Without Test article | 2 | N/A |
NOTE: Cells treated with inhibitors will be pre-incubated with inhibitor for 30 minutes prior to assay onset. Cells used for testing with no inhibitors will be pre-incubated with blank transport buffer for 30 minutes prior to assay onset.
- LC/MS/MS analysis of cladribine in donor and receiver samples only with a minimum 6-point standard curve
- At least 60% standards must be accurate to within ±20%, except at the Lower Limit of Quantitation (LLOQ), where ±25% will be considered acceptable
- Monolayer integrity confirmed via post-experiment Lucifer Yellow permeability assessment
Assay QC
- The quality of the monolayer batch is verified using control test articles before the monolayers are released for use
- The quality of each monolayer used in the assay is verified by calculating the Papp for the control test article lucifer yellow post-experiment
Objectives
To determine if the Customer’s test article is a potential inhibitor of the multi drug resistance protein 2 (MRP2) transporter using MRP2-expressing membrane vesicles
Required from Customer
- Test article either in powder form (1 mg) or stock solution (100 µL of 10 mM DMSO)
- Molecular weight of the test article and its salt form
- Relevant solubility of test article
- The MSDS and handling and storage information, e.g., light sensitive, store at -20ºC, etc.
Deliverables
Express Plus report including:
- Materials and methods
- Results: Net influx rate of probe substrate in the presence and absence of test article
Assay System
- MRP2-expressing membrane vesicles
- MRP2 probe substrate: Estradiol 17β-glucuronide
- 96-well format at 37°C
Assay Conditions
Influx Assessment of Probe Substrate in the Presence of Test article (Single Concentration)
- Assay is performed using MRP2-expressing vesicles
- Assay buffer: Hepes Influx Buffer (10mM Hepes-Tris, pH 7.4)
- Treatment groups:
| Treatment | Replicates | Probe Substrate | Concentration of Inhibitor | Incubation Duration |
|---|---|---|---|---|
| With test article and ATP | 2 | Estradiol 17β-glucuronide (50 µM) | 10 µM | 30 min |
| With test article and AMP | 2 | 10 µM | 30 min | |
| Negative Control (solvent only) with ATP | 2 | N/A | 30 min | |
| Negative Control (solvent only) with AMP | 2 | N/A | 30 min |
- Samples are processed and transferred to a 96-well plate
- Radioactivity is measured using a scintillation counter
- Determination of percent inhibition using influx rate
Net Influx Rate (NIR) = Uptake activity with ATP – Uptake activity with AMP
Percent Inhibition =
Where,
TC: Test article
Optional Positive Control Performed in Parallel, additional fees apply
- Influx Assessment of Probe Substrate in the Presence of a known inhibitor (Single Concentration)
- Each treatment run in duplicate (n=2)
|
Treatment |
Replicates |
Probe Substrate |
Concentration of Inhibitor |
Incubation Duration |
|---|---|---|---|---|
|
Positive Control (MK571) with ATP |
2 |
Estradiol 17β-glucuronide (50 µM) |
50 mM |
30 min |
|
Positive Control (MK571) with AMP |
2 |
50 mM |
30 min |
- Determine net influx rate of probe substrate in the presence and absence of inhibitor. Calculate percent inhibition.
- Acceptance criteria:
- Percent inhibition > 50% when co-incubated with a known inhibitor.
Objectives
To determine if the Customer’s test article is a potential inhibitor of the multi drug resistance protein 2 (MRP2) transporter using MRP2-expressing membrane vesicles
Required from Customer
- Test article either in powder form (5 mg) or stock solution (100 µL of 100 mM DMSO)
- Molecular weight of the test article and its salt form
- Relevant solubility of test article
- The MSDS and handling and storage information, e.g., light sensitive, store at -20ºC, etc.
Deliverables
- Express Plus report including:
- Materials and methods
- Results: Determination of IC50 for test article
Assay System
- MRP2-expressing membrane vesicles
- MRP2 probe substrate: Estradiol 17β-glucuronide
- 96-well format at 37°C
Assay Conditions
- Influx assessment of probe substrate in the presence and absence of test article (IC50 Determination)
- Assay is performed using MRP2 -expressing vesicles
- Assay buffer: Hepes Influx Buffer (10mM Hepes-Tris, pH 7.4)
Treatment Groups
| Treatment | Replicates | Probe Substrate | Concentration of Inhibitor | Incubation Duration |
|---|---|---|---|---|
| With test article and ATP | 2 | Estradiol 17β-glucuronide (50 µM) | 6 concentrations | 30 min |
| With test article and AMP | 2 | 6 concentrations | 30 min | |
| Negative Control (solvent only) with ATP | 2 | N/A | 30 min | |
| Negative Control (solvent only) with AMP | 2 | N/A | 30 min | |
| Positive Control (MK571) with ATP | 2 | 50 µM | 30 min | |
| Positive Control (MK571) with AMP | 2 | 50 µM | 30 min |
- Samples are processed and transferred to a 96-well plate
- Radioactivity is measured using a scintillation counter
- Percent activity remaining determined for the test and control compound
Net Influx Rate (NIR) = Uptake activity with ATP – Uptake activity with AMP
Percent activity remaining =
- Positive control acceptance criteria: Percent inhibition > 50% when co-incudated with a known inhibitor
- Determination of IC50 by nonlinear regression using GraphPad Prism (version 5.0)
Y =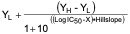
X = logarithm of the nominal concentration of test article
Y = value of the average percent remaining of the transporter at a given concentration
YL = the lowest response; this value is set to zero (0)
YH = the highest response at the lowest concentration of the inhibitor
Substrate
This assay is used to determine the interaction of a test article with P-gp using MDR1- MDCK cell monolayers with and without a P-gp inhibitor
Required from Sponsor
- Either a minimum of 100 µL of test article at 10 mM in DMSO or 2 mg of powder
- Molecular weight of the test article and its salt form
- MSDS or handling and storage information e.g., light sensitive, store at -20°C, etc.
Deliverables
- Cell batch QC results
- The percent recovery of the test article from the assay wells containing MDR1-MDCK monolayers
- The apparent permeability (Papp) of the test article in both directions in the presence and absence of inhibitor
- The efflux ratio in the presence and absence of inhibitor
- The brain penetration potential of a test article classified as either low, moderate, or high using the following criteria
- Low: Papp A→B < 3.0 x 10-6 cm/s
- Low: Papp A→B ≥ 3.0 x 10-6 cm/s, Efflux ≥ 10
- Moderate: Papp A→B ≥ 3.0 x 10-6 cm/s, 10 > Efflux ≥ 3.0
- High: Papp A→B ≥ 3.0 x 10-6 cm/s, Efflux < 3.0
- P-gp substrate classification:
- Positive: Efflux ratio ≥2.0 in the absence of inhibitor and corrected efflux ratio reduced by ≥50% in the presence of inhibitor
- Negative: Efflux ratio ≥2.0 in the absence of inhibitor and corrected efflux ratio reduced by <50% in the presence of inhibitor
- Negative: Efflux ratio < 2.0 in both the absence and presence of inhibitor
Substrate
- Test article at 5 µM in HBSSg with maximum DMSO concentration ≤ 0.8%
Assay System
- Confluent monolayers of MDR1-MDCK cells, 7 to 12 days old
Assay Conditions
- Bidirectional permeability of the test article in MDR1-MDCK cells in the presence and absence of inhibitor
- Transport buffer: HBSSg, pH 7.4 ± 0.2
- Receiver well containing 1% BSA
- Apical and basolateral side at pH 7.4
- Dose two cell monolayers for each direction(n=2), in the presence and absence of inhibitor
- Dose apical side for (A→B) assessment
- Dose basolateral side for (B→A) assessment
- Sample from both apical and basolateral sides at 120 minutes
- Determine the concentration of test article using a generic LC-MS/MS method with a minimum 6-point calibration curve
Assay QC
- The quality of the monolayer batch is verified using control compounds before the monolayers are released for use
- The quality of each monolayer used in the assay is verified by calculating the Papp for the control article, lucifer yellow, dosed post-experimentally
Notes
- The results from this assay are sent to the sponsor in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference articles.
- This screening assay does not provide definitive information about the P-gp article interactions or the apparent Km.
- Assay conditions with the inhibitor include:
- A 30 minute pre-incubation with Valspodar
- Assay conditions without the inhibitor include:
- A 30 minute pre-incubation with buffer
- Digoxin can be run as an optional positive control
- Dosed at 10 µM
- BSA is not included in the receiver chamber
This assay is used to identify active uptake of a test compound by PepT1, using the Caco-2 cell monolayer system
Required from Customer
- Either a minimum of 300 µL of test compound at 10 mM in DMSO, or 5 mg of powder
- Exact molecular mass of test compound and its salt form
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Percent recovery of test compound from Transwell® wells containing Caco-2 cell monolayers
- Apparent permeability (Papp) of test compound in the apical-to-basolateral (A to B) direction in the presence and absence of a PepT1 inhibitor
- PeptT1 interaction potential of test compound classified as either positive or negative
Substrate
- Test compound at 5 µM in modified Hanks’ buffer (HBSSg) with final DMSO concentration <0.8%
Assay System
- Confluent monolayers of PepT1-induced Caco-2 cells, 21-28 days old, in Transwell dual-chamber plates
Assay Conditions
- Receiver well with 1% BSA in modified Hank’s buffer (HBSSg)
- Pre-incubate monolayers with assay buffer (apical pH 5.0, basolateral pH 7.4), with or without GlySar, for 30 minutes prior to initiation of the assay
- Dose duplicate monolayers (N=2) with test compound on the apical side only
- Dose two other monolayers (N=2) with test compound and a PepT1 inhibitor, GlySar, on the apical side only
- Sample both apical and basolateral sides at 120 minutes
- Determine the concentration of test compound using a generic LC-MS/MS method with a minimum 4-point calibration curve
Assay QC
- Verify the quality of the cell monolayer batch using control compounds before the monolayers are released for use
- Verify the quality of each monolayer by pre-experiment TEER and Papp of a co-dosed control compound
Notes
- The results from this assay are provided to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
Options
The customer can request: that a positive control (valacyclovir) be run in parallel
This assay is used to determine the interaction of a test compound with BCRP using BCRP-MDCK cell monolayers with and without a BCRP inhibitor
Required from Customer
- Either a minimum of 300 µL of test compound at 10 mM in DMSO, or 5 mg of powder
- Exact molecular mass of test compound and its salt form
- Solubility of test compound in Hanks’ buffer
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- The percent recovery of the test compound from Transwell® wells containing cell monolayers
- The apparent permeability (Papp) in both directions with and without inhibitor
- The efflux ratio (Papp B to A)/(Papp A to B) with and without inhibitor
- BCRP substrate classification:
- Positive when efflux ratio ≥3.0 in the absence of inhibitor and relative efflux ratio ≥2.0
- Negative when efflux ratio ≥3.0 in the absence of inhibitor and relative efflux ratio <2.0, or when efflux ratio <3.0 in the absence and presence of inhibitor
- Requires further study when the effux ratio ≥3.0 in the absence and presence of inhibitor
Substrate
- Test compound at 5 µM in HBSSg with maximum DMSO concentration not greater than 1%
Assay System
- Confluent monolayers of BCRP-MDCK cells, 7 to 11 days old
Assay Conditions
- Receiver well with 1% BSA in modified Hanks buffer (HBSSg)
- Apical and basolateral side at pH 7.4 ± 0.2
- Assay run in duplicate (N=2)
- Single test compound concentration at 5 µM
- Dose apical side for A to B assessment and basolateral side for B to A assessment
- Sample both donor and receiver sides at 120 minutes
- Determine the concentrations of test compound using a generic LC-MS/MS method with a minimum 6-point calibration curve
Assay QC
- The quality of the cell monolayer batch is verified using control compounds before the monolayers are used
- The quality of each monolayer used in the assay is verified by a TEER measurement and by calculating the Papp for a control compound
Notes
- The results from this assay are sent to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- Monolayer integrity will be verified by a pre-experimental TEER.
- This screening assay does not provide definitive information about the BCRP compound interactions or the apparent Km.
- Assay conditions with the inhibitor include: a 30 minute pre-incubation with Ko143
- Assay conditions without the inhibitor include: a 30 minute pre-incubation with buffer
This assay is used to determine the interaction of a test compound with BCRP using Caco-2 cell monolayers with and without a BCRP inhibitor
Required from Customer
- Either a minimum of 300 µL of test compound at 10 mM in DMSO, or 5 mg of powder
- Exact molecular mass of test compound and its salt form
- Solubility of test compound in Hanks’ buffer
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- The percent recovery of the test compound from Transwell® wells containing cell monolayers
- The apparent permeability (Papp) in both directions with and without inhibitor
- The efflux ratio (Papp B to A)/(Papp A to B) with and without inhibitor
- BCRP substrate classification:
- Positive when efflux ratio ≥3.0 in the absence of inhibitor and relative efflux ratio ≥2.0
- Negative when efflux ratio ≥3.0 in the absence of inhibitor and relative efflux ratio <2.0, or when efflux ratio <3.0 in the absence and presence of inhibitor
- Requires further study when the effux ratio ≥3.0 in the absence and presence of inhibitor
Substrate
- Test compound at 5 µM in HBSSg with maximum DMSO concentration not greater than 1%
Assay System
- Confluent monolayers of Caco-2 cells, 21 to 28 days old
Assay Conditions
- Receiver well with 1% BSA in modified Hanks buffer (HBSSg)
- Apical and basolateral side at pH 7.4 ± 0.2
- Assay run in duplicate (N=2)
- Single test compound concentration at 5 µM
- Dose apical side for A to B assessment and basolateral side for B to A assessment
- Sample both donor and receiver sides at 120 minutes
- Determine the concentrations of test compound using a generic LC-MS/MS method with a minimum 6-point calibration curve
Assay QC
- The quality of the cell monolayer batch is verified using control compounds before the monolayers are used
- The quality of each monolayer used in the assay is verified by a TEER measurement and by calculating the Papp for a control compound
Notes
- The results from this assay are sent to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- Monolayer integrity will be verified by a pre-experimental TEER.
- This screening assay does not provide definitive information about the BCRP compound interactions or the apparent Km.
- Assay conditions with the inhibitor include: a 30 minute pre-incubation with Ko143
- Assay conditions without the inhibitor include: a 30 minute pre-incubation with buffer
This assay is used to determine the interaction of a test compound with P-gp using Caco-2 cell monolayers with and without a P-gp inhibitor.
Required from Customer
- Either a minimum of 300 µL of test compound at 10 mM in DMSO, or 5 mg of powder
- Exact molecular mass of test compound and its salt form
- Solubility of test compound in Hanks’ buffer
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- The percent recovery of the test compound from Transwell® wells containing cell monolayers
- The apparent permeability (Papp) in both directions with and without inhibitor
- The efflux ratio (Papp B to A)/(Papp A to B) with and without inhibitor
- P-gp substrate classification:
- Positive when efflux ratio ≥3.0 in the absence of inhibitor and relative efflux ratio ≥2.0
- Negative when efflux ratio ≥3.0 in the absence of inhibitor and relative efflux ratio <2.0, or when efflux ratio <3.0 in the absence and presence of inhibitor
- Requires further study when the effux ratio ≥3.0 in the absence and presence of inhibitor
Substrate
- Test compound at 5 µM in HBSSg with maximum DMSO concentration not greater than 1%
Assay System
- Confluent monolayers of Caco-2 cells, 21 to 28 days old
Assay Conditions
- Receiver well with 1% BSA in modified Hanks buffer (HBSSg)
- Apical and basolateral side at pH 7.4 ± 0.2
- Assay run in duplicate (N=2)
- Single test compound concentration at 5 µM
- Dose apical side for A to B assessment and basolateral side for B to A assessment
- Sample both donor and receiver sides at 120 minutes
- Determine the concentrations of test compound using a generic LC-MS/MS method with a minimum 6-point calibration curve
Assay QC
- The quality of the cell monolayer batch is verified using control compounds before the monolayers are used
- The quality of each monolayer used in the assay is verified by a TEER measurement and by calculating the Papp for a control compound
Notes
- The results from this assay are sent to the customer in the ExpressPlus report format, which may include graphical representations of data and comparison with historical data for reference compounds.
- Monolayer integrity will be verified by a pre-experimental TEER.
- This screening assay does not provide definitive information about the P-gp compound interactions or the apparent Km.
- Assay conditions with the inhibitor include: a 30 minute pre-incubation with Valspodar
- Assay conditions without the inhibitor include: a 30 minute pre-incubation with buffer
This assay uses OAT1-transfected HEK293 cells to assess whether the test compound is a potential substrate of the hepatic uptake transporter OAT1
Required from Customer
- Either a minimum of 300 µL of test compound at 10 mM in DMSO, or 5 mg of powder
- Exact molecular mass of test compound and its salt form
- Solubility of test compound in Hanks’ buffer
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Normalized uptake of test compound at each concentration in OAT1-transfected and vector control cells
- Determination of substrate classification: positive when the ratio of the influx rates in OAT1-transfected cells vs. vector control cells >2.0
Substrate
- Test compound at 0.5 and 5 µM in HBSSg (pH 7.4) with maximum DMSO concentration not greater than 1%
Assay System
- OAT1-transfected HEK293 cells and vector control cells cultured in 24-well plates
Assay Conditions
- Measure uptake of test article in-transfected and vector control cells
- Two concentrations of test article and two sampling time points
- Treatments per transporter as follows (repeated for each concentration and incubation duration):
- Transfected cells with test article
- Vector control cells with test article
- Perform assay in duplicate (N=2 per treatment)
- Wells are equilibrated with blank buffer containing 1% BSA prior to initiating the assay
- Buffer is aspirated and replaced with test article dosing solution
- Incubate cells for 2 and 10 min at 37°C
- Terminate the incubation and lyse the cells
- Analyze cell lysate using LC-MS/MS to determine test article concentration
- Determine protein concentration in cell lysate and normalize probe substrate concentration to protein content
Batch QC
- The quality of the cell batch is verified before the cells are used (uptake rate of a probe substrate in OAT1-transfected vs. vector control cells)
Options The customer can request:
- An in-study positive control for an additional fee
Notes
- The results from this assay are sent to the customer in the ExpressPlus report format, which may include graphical representations of data.
This assay uses OAT3-transfected HEK293 cells to assess whether the test compound is a potential substrate of the hepatic uptake transporter OAT3
Required from Customer
- Either a minimum of 300 µL of test compound at 10 mM in DMSO, or 5 mg of powder
- Exact molecular mass of test compound and its salt form
- Solubility of test compound in Hanks’ buffer
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Normalized uptake of test compound at each concentration in OAT3-transfected and vector control cells
- Determination of substrate classification: positive when the ratio of the influx rates in OAT3-transfected cells vs. vector control cells >2.0
- Substrate
- Test compound at 0.5 and 5 µM in HBSSg (pH 7.4) with maximum DMSO concentration not greater than 1%
Assay System
- OAT3-transfected HEK293 cells and vector control cells cultured in 24-well plates
Assay Conditions
- Measure uptake of test article in-transfected and vector control cells
- Two concentrations of test article and two sampling time points
- Treatments per transporter as follows (repeated for each concentration and incubation duration):
- Transfected cells with test article
- Vector control cells with test article
- Perform assay in duplicate (N=2 per treatment)
- Wells are equilibrated with blank buffer containing 1% BSA prior to initiating the assay
- Buffer is aspirated and replaced with test article dosing solution
- Incubate cells for 2 and 10 min at 37°C
- Terminate the incubation and lyse the cells
- Analyze cell lysate using LC-MS/MS to determine test article concentration
- Determine protein concentration in cell lysate and normalize probe substrate concentration to protein content
Batch QC
- The quality of the cell batch is verified before the cells are used (uptake rate of a probe substrate in OAT3-transfected vs. vector control cells)
Options The customer can request:
- An in-study positive control for an additional fee
Notes
- The results from this assay are sent to the customer in the ExpressPlus report format, which may include graphical representations of data.
This assay uses OCT1-transfected HEK293 cells to assess whether the test compound is a potential substrate of the hepatic uptake transporter OCT1.
Required from Customer
- Either a minimum of 300 µL of test compound at 10 mM in DMSO, or 5 mg of powder
- Exact molecular mass of test compound and its salt form
- Solubility of test compound in Hanks’ buffer
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Normalized uptake of test compound at each concentration in OCT1-transfected and vector control cells
- Determination of substrate classification: positive when the ratio of the influx rates in OCT1-transfected cells vs. vector control cells >2.0
Substrate
- Test compound at 0.5 and 5 µM in HBSSg (pH 7.4) with maximum DMSO concentration not greater than 1%
Assay System
- OCT1-transfected HEK293 cells and vector control cells cultured in 24-well plates
Assay Conditions
- Measure uptake of test article in-transfected and vector control cells
- Two concentrations of test article and two sampling time points
- Treatments per transporter as follows (repeated for each concentration and incubation duration):
- Transfected cells with test article
- Vector control cells with test article
- Perform assay in duplicate (N=2 per treatment)
- Wells are equilibrated with blank buffer containing 1% BSA prior to initiating the assay
- Buffer is aspirated and replaced with test article dosing solution
- Incubate cells for 2 and 10 min at 37°C
- Terminate the incubation and lyse the cells
- Analyze cell lysate using LC-MS/MS to determine test article concentration
- Determine protein concentration in cell lysate and normalize probe substrate concentration to protein content
Batch QC
- The quality of the cell batch is verified before the cells are used (uptake rate of a probe substrate in OCT1-transfected vs. vector control cells)
Options The customer can request:
- An in-study positive control for an additional fee
Notes
- The results from this assay are sent to the customer in the ExpressPlus report format, which may include graphical representations of data.
This assay uses OCT2-transfected HEK293 cells to assess whether the test compound is a potential substrate of the hepatic uptake transporter OCT2.
Required from Customer
- Either a minimum of 300 µL of test compound at 10 mM in DMSO, or 5 mg of powder
- Exact molecular mass of test compound and its salt form
- Solubility of test compound in Hanks’ buffer
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Normalized uptake of test compound at each concentration in OCT2-transfected and vector control cells
- Determination of substrate classification: positive when the ratio of the influx rates in OCT2-transfected cells vs. vector control cells >2.0
Substrate
- Test compound at 0.5 and 5 µM in HBSSg (pH 7.4) with maximum DMSO concentration not greater than 1%
Assay System
- OCT2-transfected HEK293 cells and vector control cells cultured in 24-well plates
Assay Conditions
- Measure uptake of test article in-transfected and vector control cells
- Two concentrations of test article and two sampling time points
- Treatments per transporter as follows (repeated for each concentration and incubation duration):
- Transfected cells with test article
- Vector control cells with test article
- Perform assay in duplicate (N=2 per treatment)
- Wells are equilibrated with blank buffer containing 1% BSA prior to initiating the assay
- Buffer is aspirated and replaced with test article dosing solution
- Incubate cells for 2 and 10 min at 37°C
- Terminate the incubation and lyse the cells
- Analyze cell lysate using LC-MS/MS to determine test article concentration
- Determine protein concentration in cell lysate and normalize probe substrate concentration to protein content
Batch QC
- The quality of the cell batch is verified before the cells are used (uptake rate of a probe substrate in OCT2-transfected vs. vector control cells)
Options The customer can request:
- An in-study positive control for an additional fee
Notes
- The results from this assay are sent to the customer in the ExpressPlus report format, which may include graphical representations of data.
This assay uses OATP1B1-transfected HEK293 cells to assess whether the test compound is a potential substrate of the hepatic uptake transporter OATP1B1
Required from Customer
- Either a minimum of 300 µL of test compound at 10 mM in DMSO, or 5 mg of powder
- Exact molecular mass of test compound and its salt form
- Solubility of test compound in Hanks’ buffer
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc
Deliverables
- Normalized uptake of test compound at each concentration in OATP1B1-transfected and vector control cells
- Determination of substrate classification: positive when the ratio of the influx rates in OATP1B1-transfected cells vs. vector control cells >2.0
Substrate
- Test compound at 0.5 and 5 µM in HBSSg (pH 7.4) with maximum DMSO concentration not greater than 1%
Assay System
- OATP1B1-transfected HEK293 cells and vector control cells cultured in 24-well plates
Assay Conditions
- Measure uptake of test article in-transfected and vector control cells
- Two concentrations of test article and two sampling time points
- Treatments per transporter as follows (repeated for each concentration and incubation duration):
- Transfected cells with test article
- Vector control cells with test article
- Perform assay in duplicate (N=2 per treatment)
- Wells are equilibrated with blank buffer containing 1% BSA prior to initiating the assay
- Buffer is aspirated and replaced with test article dosing solution
- Incubate cells for 2 and 10 min at 37°C
- Terminate the incubation and lyse the cells
- Analyze cell lysate using LC-MS/MS to determine test article concentration
- Determine protein concentration in cell lysate and normalize probe substrate concentration to protein content
Batch QC
- The quality of the cell batch is verified before the cells are used (uptake rate of a probe substrate in OATP1B1-transfected vs. vector control cells)
Options
The customer can request:
- An in-study positive control for an additional fee
Notes
- The results from this assay are sent to the customer in the ExpressPlus report format, which may include graphical representations of data.
This assay uses OATP1B3-transfected HEK293 cells to assess whether the test compound is a potential substrate of the hepatic uptake transporter OATP1B3
Required from Customer
- Either a minimum of 300 µL of test compound at 10 mM in DMSO, or 5 mg of powder
- Exact molecular mass of test compound and its salt form
- Solubility of test compound in Hanks’ buffer
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Normalized uptake of test compound at each concentration in OATP1B3-transfected and vector control cells
- Determination of substrate classification: positive when the ratio of the influx rates in OATP1B3-transfected cells vs. vector control cells >2.0
Substrate
- Test compound at 0.5 and 5 µM in HBSSg (pH 7.4) with maximum DMSO concentration not greater than 1%
Assay System
- OATP1B3-transfected HEK293 cells and vector control cells cultured in 24-well plates
Assay Conditions
- Measure uptake of test article in-transfected and vector control cells
- Two concentrations of test article and two sampling time points
- Treatments per transporter as follows (repeated for each concentration and incubation duration):
- Transfected cells with test article
- Vector control cells with test article
- Perform assay in duplicate (N=2 per treatment)
- Wells are equilibrated with blank buffer containing 1% BSA prior to initiating the assay
- Buffer is aspirated and replaced with test article dosing solution
- Incubate cells for 2 and 10 min at 37°C
- Terminate the incubation and lyse the cells
- Analyze cell lysate using LC-MS/MS to determine test article concentration
- Determine protein concentration in cell lysate and normalize probe substrate concentration to protein content
Batch QC
- The quality of the cell batch is verified before the cells are used (uptake rate of a probe substrate in OATP1B3-transfected vs. vector control cells)
Options | The customer can request:
- An in-study positive control for an additional fee
Notes
- The results from this assay are sent to the customer in the ExpressPlus report format, which may include graphical representations of data.
This assay uses MATE1-transfected HEK293 cells to assess whether the test compound is a potential substrate of the hepatic uptake transporter MATE1
Required from Customer
- Either a minimum of 300 µL of test compound at 10 mM in DMSO, or 5 mg of powder
- Exact molecular mass of test compound and its salt form
- Solubility of test compound in Hanks’ buffer
- MSDS or handling and storage information, e.g., light sensitive, store at -20°C, etc.
Deliverables
- Normalized uptake of test compound at each concentration in MATE1-transfected and vector control cells
- Determination of substrate classification: positive when the ratio of the influx rates in MATE1-transfected cells vs. vector control cells >2.0
Substrate
- Test compound at 0.5 and 5 µM in HBSSg (pH 8.5) with maximum DMSO concentration not greater than 1%
Assay System
- MATE1-transfected HEK293 cells and vector control cells cultured in 24-well plates
Assay Conditions
- Measure uptake of test article in-transfected and vector control cells
- Two concentrations of test article and two sampling time points
- Treatments per transporter as follows (repeated for each concentration and incubation duration):
- Transfected cells with test article
- Vector control cells with test article
- Perform assay in duplicate (N=2 per treatment)
- Wells are equilibrated with blank buffer containing 1% BSA prior to initiating the assay
- Buffer is aspirated and replaced with test article dosing solution
- Incubate cells for 2 and 10 min at 37°C
- Terminate the incubation and lyse the cells
- Analyze cell lysate using LC-MS/MS to determine test article concentration
- Determine protein concentration in cell lysate and normalize probe substrate concentration to protein content
Batch QC
- The quality of the cell batch is verified before the cells are used (uptake rate of a probe substrate in MATE1-transfected vs. vector control cells)
Options The customer can request:
- An in-study positive control for an additional fee
Notes
- The results from this assay are sent to the customer in the ExpressPlus report format, which may include graphical representations of data.
This assay uses MATE2K-transfected HEK293 cells to assess whether the test compound is a potential substrate of the hepatic uptake transporter MATE2K
Required from Customer
- Either a minimum of 300 µL of test compound at 10 mM in DMSO, or 5 mg of powder
- Exact molecular mass of test compound and its salt form
- Solubility of test compound in Hanks’ buffer
- MSDS or handling and storage information, e.g., light-sensitive, store at -20°C, etc
Deliverables
- Normalized uptake of test compound at each concentration in MATE2K-transfected and vector control cells
- Determination of substrate classification: positive when the ratio of the influx rates in MATE2K-transfected cells vs. vector control cells >2.0
Substrate
- Test compound at 0.5 and 5 µM in HBSSg (pH 8.5) with maximum DMSO concentration not greater than 1%
Assay Systems
- MATE2K-transfected HEK293 cells and vector control cells cultured in 24-well plates
Assay Condition
- Measure uptake of test article in-transfected and vector control cells
- Two concentrations of test article and two sampling time points
- Treatments per transporter as follows (repeated for each concentration and incubation duration):
- Transfected cells with test article
- Vector control cells with test article
- Perform assay in duplicate (N=2 per treatment)
- Wells are equilibrated with blank buffer containing 1% BSA prior to initiating the assay
- Buffer is aspirated and replaced with test article dosing solution
- Incubate cells for 2 and 10 min at 37°C
- Terminate the incubation and lyse the cells
- Analyze cell lysate using LC-MS/MS to determine test article concentration
- Determine protein concentration in cell lysate and normalize probe substrate concentration to protein content
Batch QC
- The quality of the cell batch is verified before the cells are used (uptake rate of a probe substrate in MATE2K-transfected vs. vector control cells)
Options
The customer can request:
- An in-study positive control for an additional fee
Notes
- The results from this assay are sent to the customer in the ExpressPlus report format, which may include graphical representations of data.
Objective
To determine if the Sponsor’s test article is a potential substrate of the hepatic bile salt export pump (BSEP) transporter using BSEP-expressing membrane vesicles
Required from Customer
- Test article either in powder form (1 mg) or stock solution (100 µL of 10 mM DMSO)
- Molecular weight of the test article and its salt form
- The MSDS and handling and storage information, e.g., light sensitive, store at -20ºC, etc.
Deliverables
Express Plus report including:
- Materials and methods
- Results
- Influx rate of the test article in BSEP-expressing membrane vesicles in the presence and absence of ATP
- BSEP substrate assessment based on calculated influx rate ratio
Assay System
- BSEP-expressing membrane vesicles
- hBSEP probe substrate: Taurocholic acid (TCA)
- 96-well format at 37°C
Assay Conditions
Influx Assessment of test article (Single Concentration)
- Assay is performed using BSEP-expressing vesicles
- Assay buffer: Hepes Influx Buffer (10mM Hepes-Tris, pH 7.4)
- Treatment groups:
| Treatment | Replicates | Incubation Duration |
|---|---|---|
| Test article (10 µM) and ATP | 2 | 30 min |
| Test article (10 µM) and AMP | 2 | 30 min |
- Optional Positive Control Performed in Parallel, additional fees apply
| Treatment | Replicates | Incubation Duration |
|---|---|---|
| Taurocholic acid (5 µM) with ATP | 2 | 30 min |
| Taurocholic acid (5 µM) with AMP | 2 | 30 min |
- Samples are processed and transferred to a 96-well plate
- Concentration is measured using LC-MS/MS
- Determination of influx rate ratio
- Influx Rate Ratio (IRR) = (Influx Rate with ATP) / (Influx Rate with AMP)
- Acceptance criteria:
- Influx rate ratio of the positive control must be > 2.0.
Objective
To determine if the Sponsor’s test article is a potential substrate of the multi drug resistance protein 2 (MRP2) transporter using MRP2-expressing membrane vesicles
Required from Customer
- Test article either in powder form (1 mg) or stock solution (100 µL of 10 mM DMSO)
- Molecular weight of the test article and its salt form
- The MSDS and handling and storage information, e.g., light sensitive, store at -20ºC, etc.
Deliverables
Express Plus report including:
- Materials and methods
- Results
- Influx rate of the test article in MRP2-expressing membrane vesicles in the presence and absence of ATP
- MRP2 substrate assessment based on calculated influx rate ratio
Assay System
- MRP2-expressing membrane vesicles
- hMRP2 probe substrate: Estradiol 17β-glucuronide
- 96-well format at 37°C
Assay Conditions
Influx Assessment of test article (Single Concentration)
- Assay is performed using MRP2-expressing vesicles
- Assay buffer: Hepes Influx Buffer (10mM Hepes-Tris, pH 7.4)
- Treatment groups:
| Treatment | Replicates | Incubation Duration |
|---|---|---|
| Test article (10 µM) and ATP | 2 | 30 min |
| Test article (10 µM) and AMP | 2 | 30 min |
- Optional Positive Control Performed in Parallel, additional fees apply
| Treatment | Replicates | Incubation Duration |
|---|---|---|
| Estradiol 17β-glucuronide (50 µM) with ATP | 2 | 30 min |
| Estradiol 17β-glucuronide (50 µM) with AMP | 2 | 30 min |
- Samples are processed and transferred to a 96-well plate
- Concentration is measured using LC-MS/MS
- Determination of influx rate ratio
- Influx Rate Ratio (IRR) = (Influx Rate with ATP) / (Influx Rate with AMP)
- Acceptance criteria:
- Influx rate ratio of the positive control must be > 2.0.
Place an Online order
To view pricing and place online orders, you must have an account and be logged in.
