Ocular Safety Assessment

Ocular Safety Assessment Services
Pharmaron’s ocular toxicology studies can be conducted under GLP (histopathology and clinical pathology) or non-GLP conditions. Our team has experience using small molecules, biologics, cell therapy, gene therapy, implants and transplants and works with rodents and non-rodents. We have on-site ACVO board-certified veterinary ophthalmologists to oversee all studies.
Ocular Disease Models
- Glaucoma
- Choroidal Neovascularization (CNV)
- Age-Related Macular Degeneration (AMD)
- Keratoconjunctivitis Sicca (KCS)
- Allergic conjunctivitis
- Anterior uveitis
- Infectious keratitis
- Neuroprotection
- Corneal wound healing
- Diabetic macular edema
Capabilities

Data and Image Capturing Capabilities
- Slit-lamp biomicroscopy with photography
- Pneumatonometery (IOP Measurement)
- Optical coherence tomography
- Surgical/operating microscope – videography
- Direct/indirect ophthalmoscopy
- Phacoemulsion
- Laser
- Specular microscopy
- Color fundus photography
- Fluorescein angiography
- Electroretinography
- Optokinetic response
- Vitrectomy

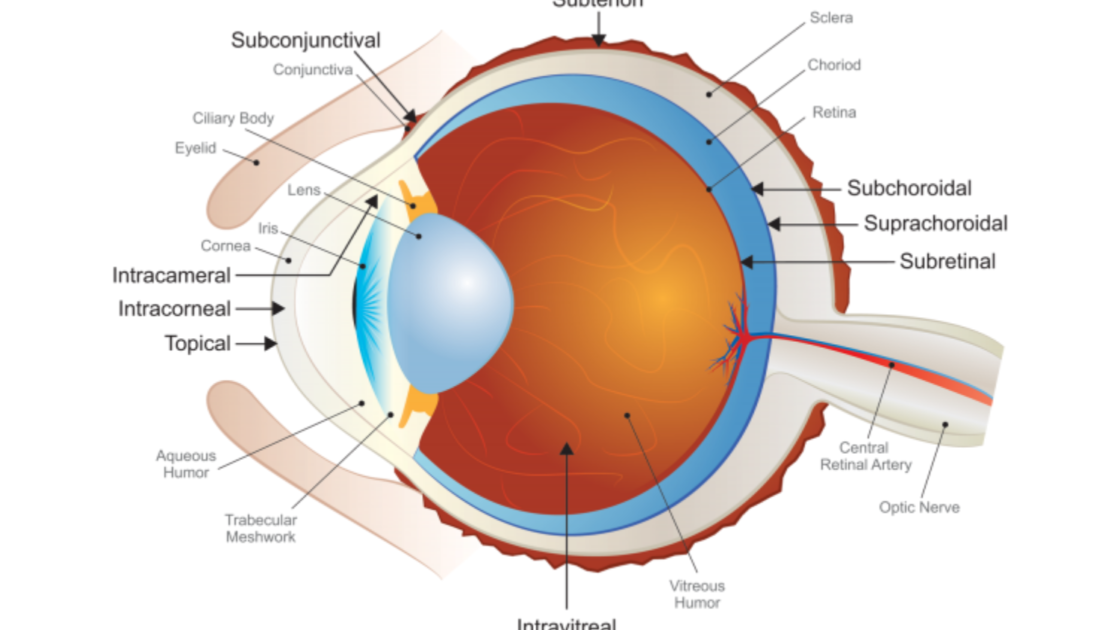
Dose Routes
- Topical
- Punctal
- Intracorneal
- Intravitreal
- Intracameral
- Periocular
- Subconjunctival
- Subtenon
- Suprachoroidal
- Subretinal
Tissue Dissection
- Lids
- Lacrimal glands
- Lacrimal ducts
- Conjunctiva
- Sclera
- Cornea
- Aqueous humor
- Iris
- Ciliary body
- Trabecular meshwork
- Lens
- Vitreous humor
- Retina
- Choroid
- Optic nerve
- Adnexa


